2017 2022 Influenza Review
2017 2022 Influenza Review
These projections may change as. Vaccine manufacturers have projected that they will supply 188 to 200 million doses of influenza vaccine for the 2021-2022 season. N Number of subjects with diary card completed. Resistance 2017-2022 Page 4of45.

Animal Influenza Virus Infections In Humans A Commentary International Journal Of Infectious Diseases
Updated guidance for the administration of seasonal influenza vaccination for healthcare staff and slidesets for the the national and childhood flu immunisation.

2017 2022 Influenza Review. B352017 Influenza Flu and Viral Respiratory Tract Infection - Testing and Isolation Precautions for Adults Guideline Page 1 of 13. The virus can infect. Europe - Seasonal Influenza Child Vaccine Market.
The 20172018 influenza season was the first season classified as a high-severity season for all age groups with high levels of outpatient clinic and emergency. These vaccines are egg based. It is recommended that quadrivalent vaccines for use in the 2021 - 2022 northern hemisphere influenza season contain the following.
Centers for Disease Control ROC Taiwan. SIV Seasonal Influenza Vaccination UK United Kingdom US United States VSS Vaccination Subsidy Scheme. What is bird flu.
Plos Computational Biology Seasonal Influenza Modelling Approaches To Capture Immunity Propagation
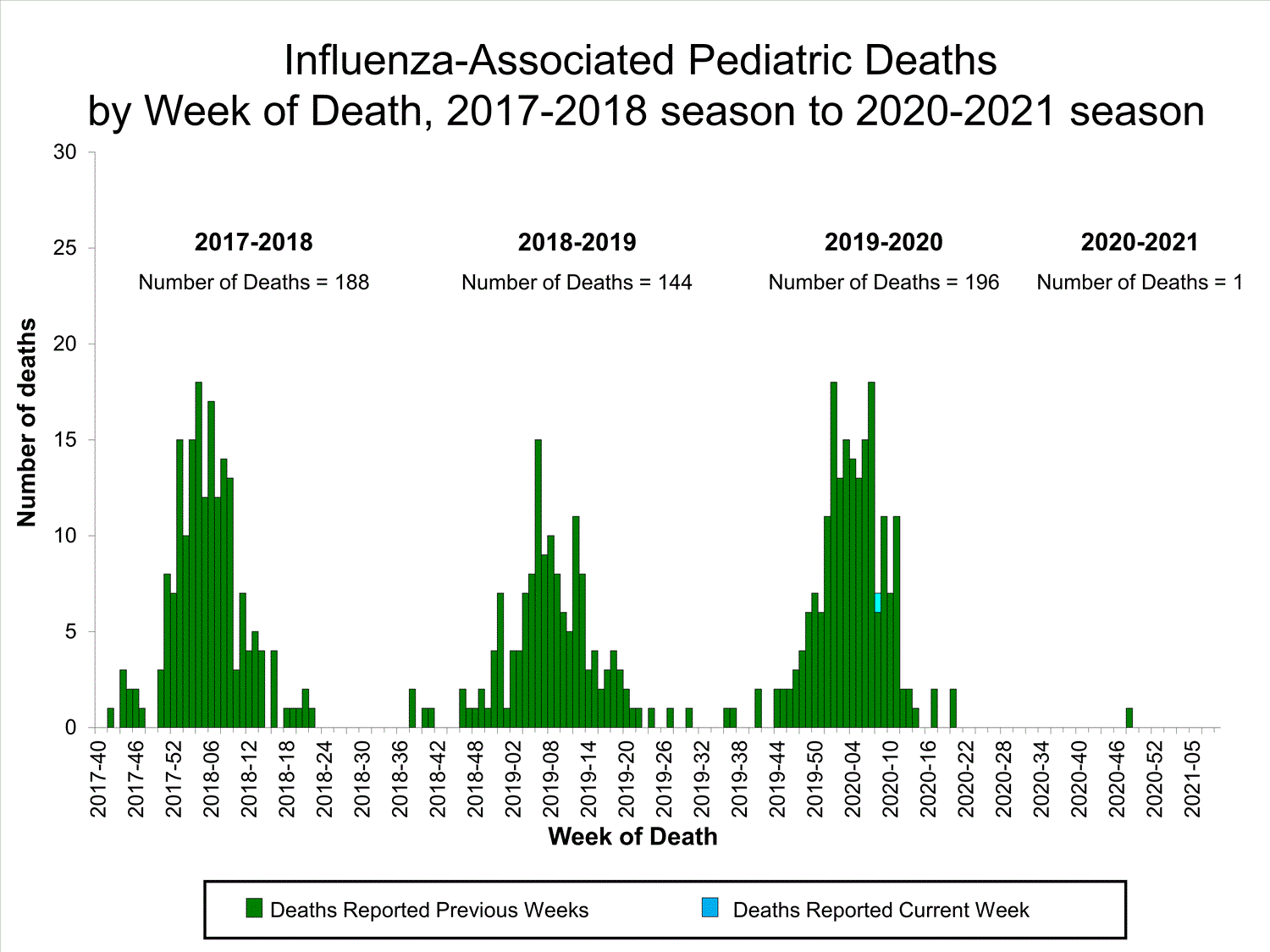
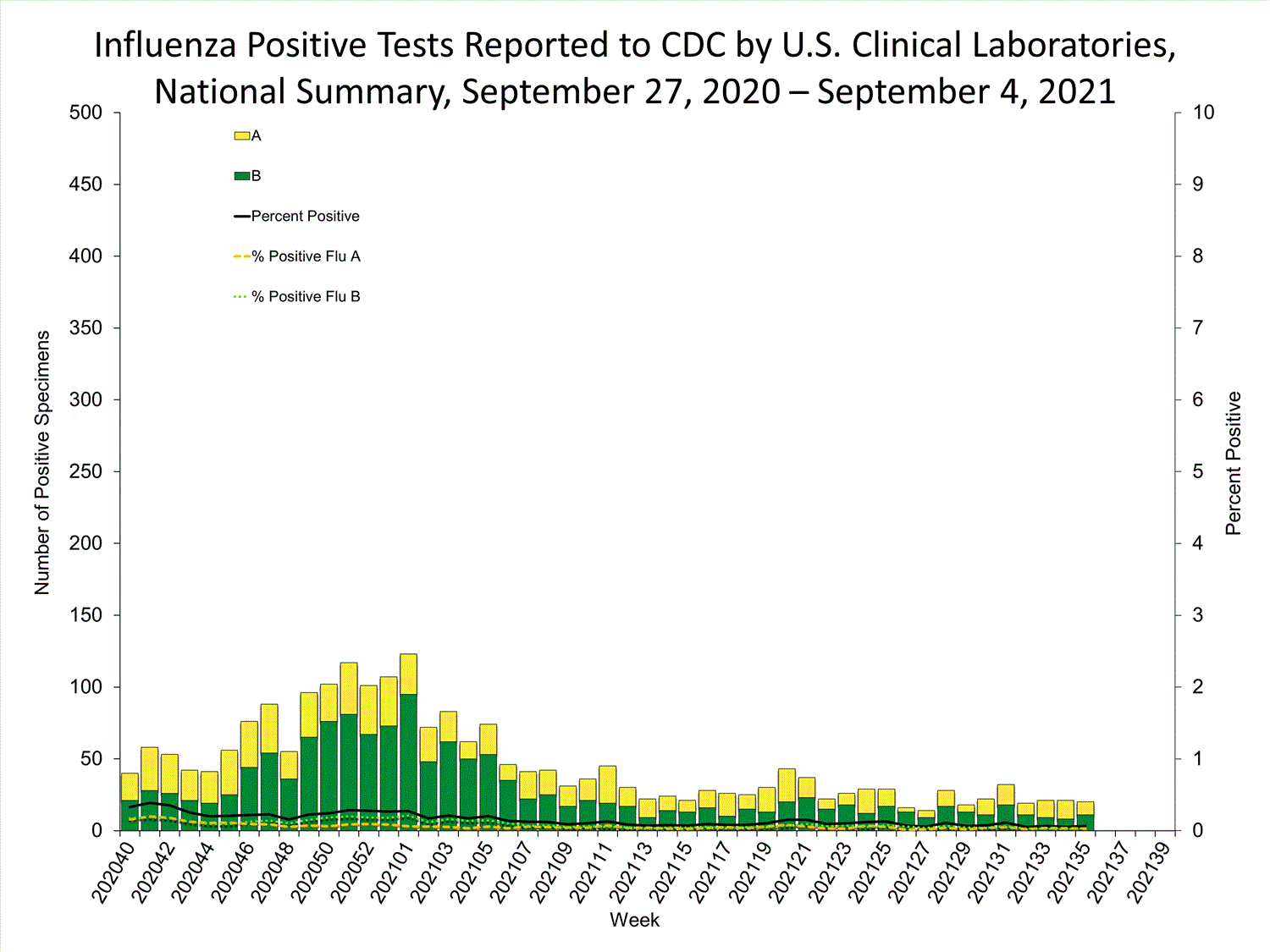
Fluview Summary Ending On March 6 2021 Cdc
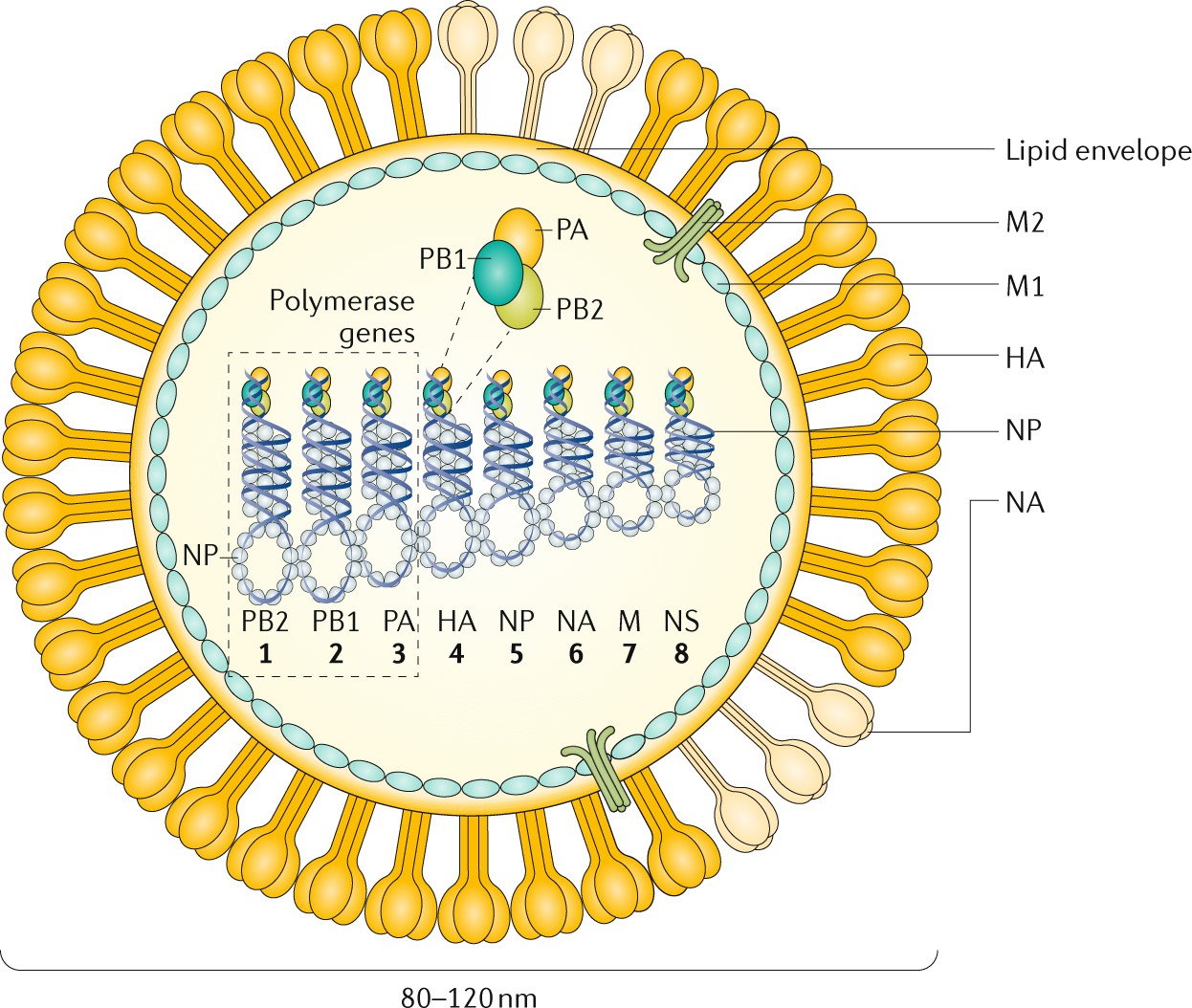
Influenza Nature Reviews Disease Primers
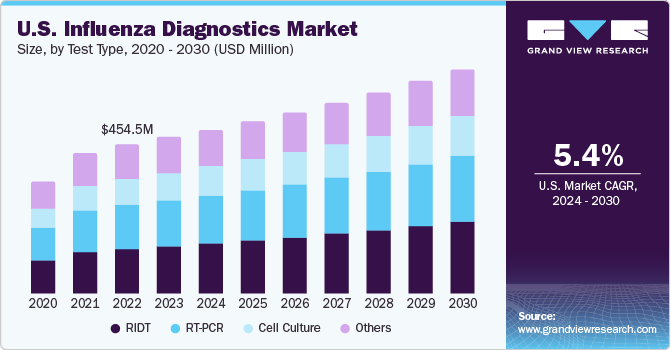
Influenza Diagnostic Market Size Share Industry Report 2013 2024
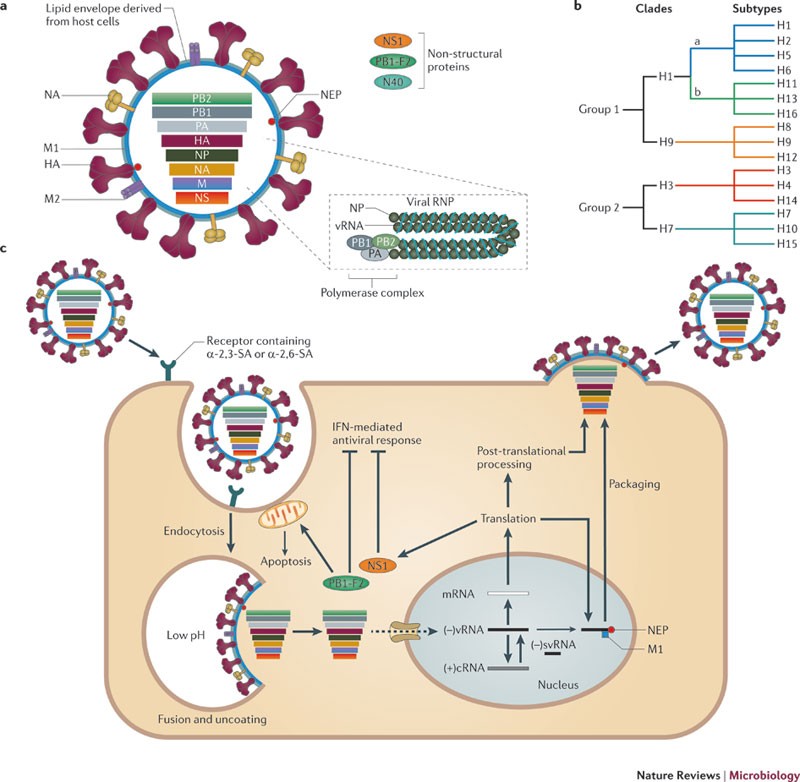
Influenza A Viruses New Research Developments Nature Reviews Microbiology
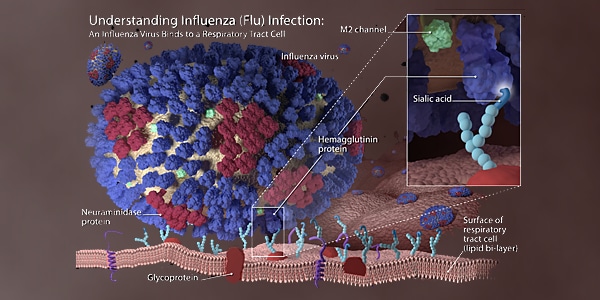
Summary Of The 2016 2017 Influenza Season Cdc
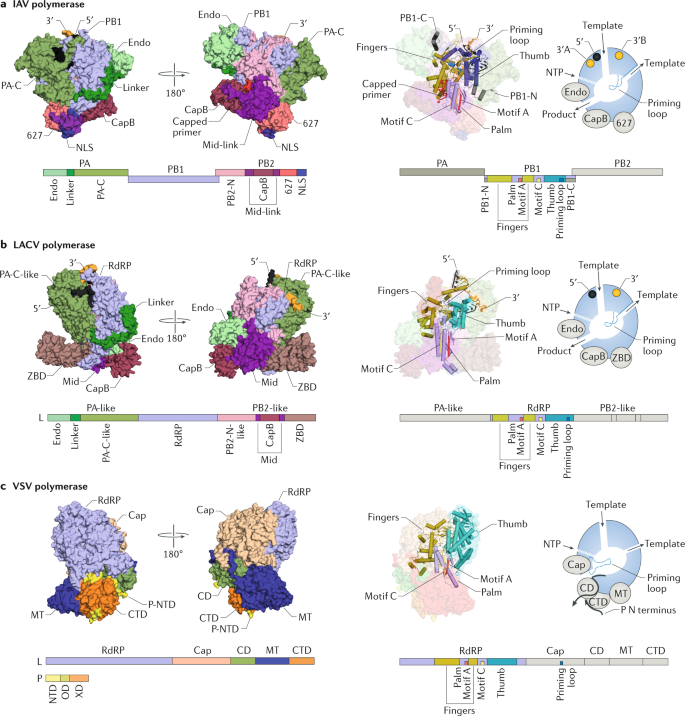
Structural Insights Into Rna Polymerases Of Negative Sense Rna Viruses Nature Reviews Microbiology

A Brief History Of Bird Flu Philosophical Transactions Of The Royal Society B Biological Sciences

Recommendations For Prevention And Control Of Influenza In Children 2018 2019 American Academy Of Pediatrics

Who Meeting Of Mid Term Review Of The Rsv Surveillance Pilot Based On The Global Influenza

Positive Phase Iii Results In High Risk Patients Expected To Support Fda Review Process For Roche S Xofluza Globaldata

A And B Strains Influvac 2021 2022 Inactivated Influenza Vaccine Abbott India Ltd Prescription Rs 900 Piece Id 22462865462


-framework.tmb-549v.png?sfvrsn=c892a140_3)


Post a Comment for "2017 2022 Influenza Review"